高得伟课题组介绍
Principal Investigator 丨 Research 丨 Publications 丨 Team
De-Wei Gao (高得伟), Assistant Professor, PI Address: School of Physical Science and Technology, ShanghaiTech University Email: gaodw@shanghaitech.edu.cn ORCiD: 0000-0002-6258-8378 Website: https://www.x-mol.com/groups/dwgao Curriculum Vitae: 2020.9-current, ShanghaiTech University, Assistant Professor, PI 2018.11-2020.9, University of California, Los Angeles (UCLA), Postdoctoral Fellow, Advisor:Prof. Yi Tang 2016.9-2018.10, The Scripps Research Institute (TSRI), Postdoctoral Fellow, Advisor:Prof. Keary Mark Engle 2011.9-2016.7, Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Sciences, Ph.D., Advisor: Prof. Shu-Li You 2007.9-2011.7, Northeast Forestry University, B.S., Advisor: Prof. Jinsong Peng Honor and Awards: Thieme Chemistry Journals Award, 2024 Shanghai Young Leading Talent, 2021 Shanghai Young Eastern Scholar, 2020 Shanghai Outstanding Graduate Student, 2016 SABIC (Saudi Basic Industries Corporation)-CAS (Chinese Academy of Sciences) Scholarship, 2016 National Scholarship for Excellent Ph.D. Students, 2015 NSFC-RSC International Symposium on Emerging Frontiers in Organic Chemistry (Outstanding Poster Prize), 2015 CAS Presidential Scholarship (Excellent Prize), 2014 |
Research |
Our group is extremely interested in the design of novel catalysts and new catalytic systems, achieving selective synthesis of highly valuable compounds. Additionally, our team intends to develop biocompatible reactions by using photocatalytic strategies. Postdoctoral Scholars and Research Assistants are available immediately in the Gao group. Please send CV, research summary and list of two references to Prof. Gao by email (gaodw@shanghaitech.edu.cn). |
Publications |
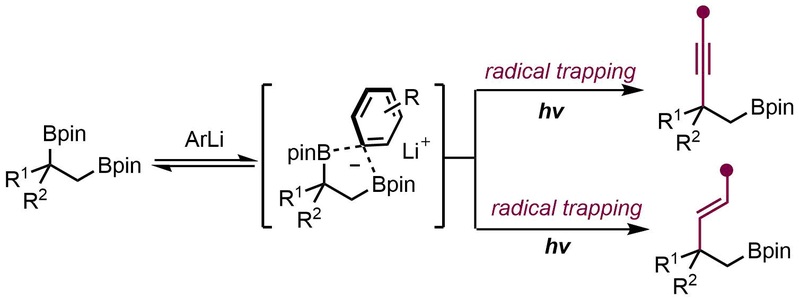
Independent Research 2024 (27) Zou, X.-Z.+; Ge, J.-F.+; Yang, Y.-X.; Huang, Y.-F.; Gao, D.-W.* Regioselective Alkynylation and Alkenylation at the More Hindered C–B Bond of 1,2-Bis(Boronic) Esters. Org. Lett. 2024, 26, 1595 (published as part of Organic Letters virtual special issue "Radical Reactions for the Construction and Transformation of Complex Molecules").
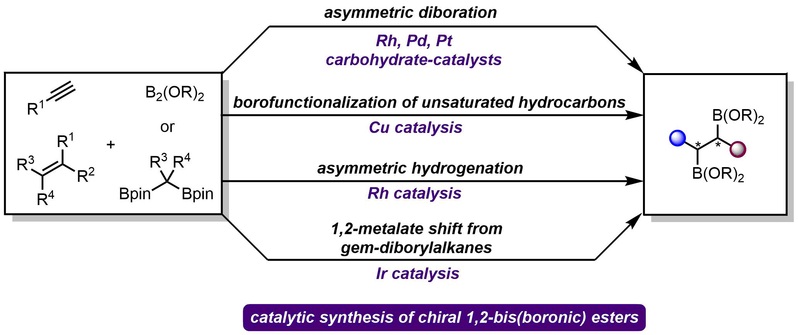
(26) Ji, C.-L.; Gao, D.-W.* Stereoselective Synthesis of 1,2-Bis(Boronates). Trends Chem. 2024, 6, 211.
(25) Ji, C.-L.; Gao, D.-W.* Recent Advances in Catalytic Asymmetric Synthesis of 1,2-Bis(Boronic) Esters. Chin. J. Org. Chem. 2024, 44, 10.6023/cjoc202312014 (Invited Review).
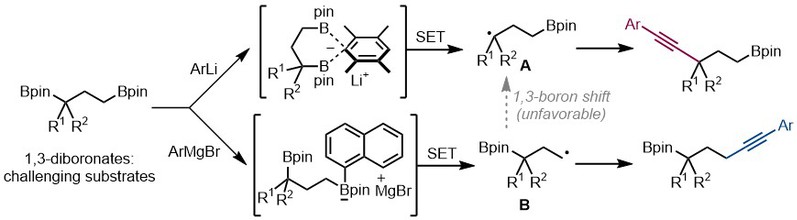
(24) Sun, Y.-W.; Gao, D.-W.* Asymmtric Synthesis of Organoborons via Ir-Catalyzed Stereoconvergent Reactions. Chem Catal. 2024, 4, 100896 (Invited Preview) (23) Chen, A.+; Ji, C.-L.+; Gao, D.-W.* Precision Alkynylation of 1,3-Bis(Boronates) Utilizing Distinct Organometallic Reagents for Regioselective Synthesis. Synlett 2024, 10.1055/a-2236-5948 (Invited Synpacts).
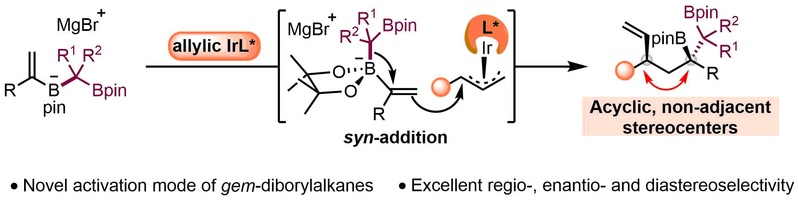
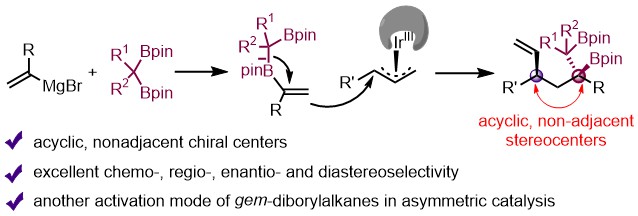
(22) Jiang, X.-M.; Ji, C.-L.; Ge, J.-F.; Zhao, J.-H.; Zhu, X.-Y.; Gao, D.-W.* Asymmetric Synthesis of Chiral 1,2-Bis(Boronic) Esters Featuring Acyclic, Non-Adjacent 1,3-Stereocenters. Angew. Chem. Int. Ed. 2024, 63, e202318441 (Highlighted in "Mechanism of the Month" of Trends in Chemistry).
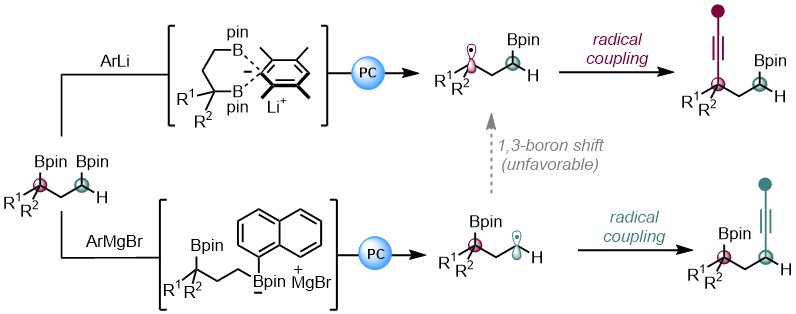
2023 (21) Chen, A; Qiao, Y.; Gao, D.-W.* Controllable Regiodivergent Alkynylation of 1,3-Bis(Boronic) Esters Activated by Distinct Organometallic Reagents. Angew. Chem. Int. Ed. 2023, 62, e202312605 (Selected as "VIP"; highlighted in Chin. J. Org. Chem. 2023, 43, 4311-4313.).
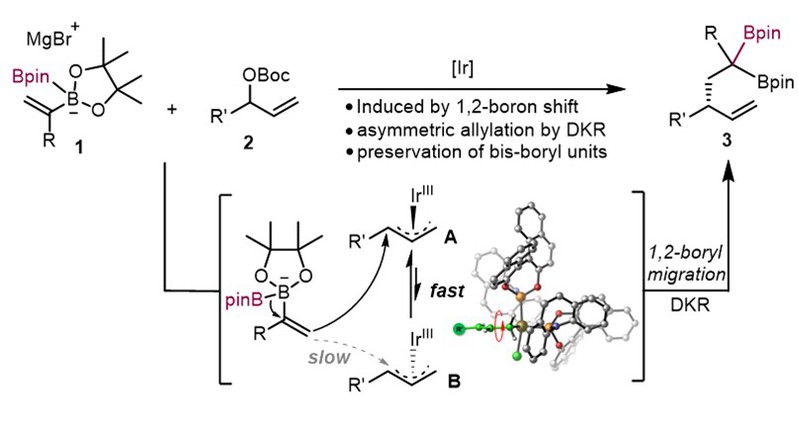
(20) Gao, D.-W. Angew. Chem. Int. Ed. 2023, 62, e202309675 (Introducing...). (19) Ge, J.-F.+; Zou, X.-Z.+; Liu, X.-R.+; Ji, C.-L.; Zhu, X.-Y.; Gao, D.-W.* Ir-Catalyzed Enantioselective Synthesis of gem-Diborylalkenes Enabled by 1,2-Boron Shift. Angew. Chem. Int. Ed. 2023, 62, e202307447. (Highlighted in Synfacts 2023, 19, 995)
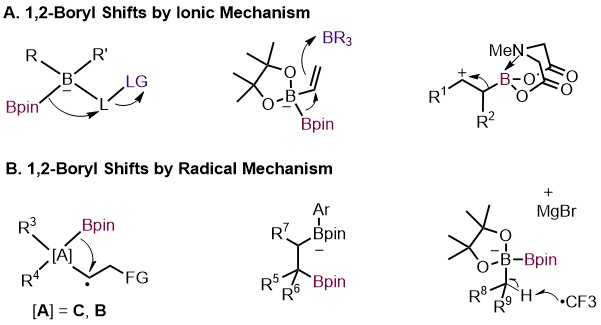
2022 (18) Jiang, X.-M.; Liu, X.-R.; Chen, A.; Zou, X.-Z.; Ge, J.-F.; Gao. D.-W.* 1,2-Boryl Migration Enables Efficient Access to Versatile Functionalized Boronates. Eur. J. Org. Chem. 2022, e202101463. (Invited review for the #NextGenOrgChem special collection.)
Before joining ShanghaiTech University (Selected Publications): (17) Gao, D.-W.†; Jamieson, C. S.†; Wang, G.†; Yan, Y.; Zhou, J.; Houk, K. N.; Tang, Y. A Polyketide Cyclase That Forms Medium-Ring Lactones. J. Am. Chem. Soc. 2021, 143, 80–84. (†Authors contributed equally) (16) Gao, D.-W.†; Gao, Y.†; Shao, H.; Qiao, T.-Z.; Wang, X.; Sanchez, B.; Chen, J. S.; Liu, P.; Engle, K. M. Cascade CuH-Catalyzed Conversion of Alkynes to Enantioenriched 1,1-Disubstituted Products. Nat. Catal. 2020, 3, 23–29.(†Authors contributed equally). (15) Gao, D.-W.; Vinogradova, E. V.; Nimmagadda, S. K.; Medina, J. M.; Xiao, Y.; Suciu, R. M.; Cravatt, B. M.; Engle, K. M. Direct Access to Versatile Electrophiles via Catalytic Oxidative Cyanation of Alkenes. J. Am. Chem. Soc. 2018, 140, 8069–8073. (Top 1 “Most Read Article” in June 2018.; Featured in Organic Chemistry Highlights.) (14) Gao, D.-W.; Xiao, Y.; Liu, M.; Liu, Z.; Karunananda, M. K.; Chen, J. S.; Engle, K. M. Catalytic, Enantioselective Synthesis of Allenyl Boronates. ACS Catal. 2018, 8, 3650–3654. (Highlighted in the Jan/Feb ChemRxiv Round-up; Highlighted in Synfacts 2018, 14, 734.; Featured in Organic Chemistry Highlights on October 22, 2018.; One of the “Most Read Articles” in March 2018.) (13) Gao, D.-W.; Gu, Q.; Zheng, C.; You, S.-L. Synthesis of Planar Chiral Ferrocenes via Transition-Metal-Catalyzed Direct C–H Bond Functionalization. Acc. Chem. Res. 2017, 50, 351–365. (12) Gao, D.-W.; Gu, Y.; Wang, S.-B.; Gu, Q.; You, S.-L. Pd(0)-Catalyzed Asymmetric C–H Alkenylation for Efficient Synthesis of Planar Chiral Ferrocenes. Organometallics 2016, 35, 3227–3233. (11) Gao, D.-W.; Gu, Q.; You, S.-L. An Enantioselective Oxidative C−H/C−H Cross-Coupling Reaction: Highly Efficient Method To Prepare Planar Chiral Ferrocenes.J. Am. Chem. Soc. 2016, 138, 2544–2547. (Highlighted by Chemical & Engineering News on March 14, 2016.) (10) Gao, D.-W.; Zheng, C.; Gu, Q.; You, S.-L. Pd-Catalyzed Highly Enantioselective Synthesis of Planar Chiral Ferrocenylpyridine Derivatives.Organometallics 2015, 34, 4618–4625. (9) Gao, D.-W.; Gu, Q.; You, S.-L. Pd(II)-Catalyzed Intermolecular Direct C−H Bond Iodination: An Efficient Approach toward the Synthesis of Axially Chiral Compounds via Kinetic Resolution. ACS Catal. 2014, 4, 2741–2745. (One of the “Most Read Articles”in July 2014.; Highlighted in ACS Catal. Spotlights.) (8) Gao, D.-W.; Yin, Q.; Gu, Q.; You, S.-L. Enantioselective Synthesis of Planar Chiral Ferrocenes via Pd(0)-Catalyzed Intramolecular Direct C−H Bond Arylation. J. Am. Chem. Soc. 2014, 136, 4841–4844. (Highlighted by Synfacts 2014, 10, 597.) (7) Gao, D.-W.; Shi, Y.-C.; Gu, Q.; Zhao, Z.-L.; You, S.-L. Enantioselective Synthesis of Planar Chiral Ferrocenes via Palladium-Catalyzed Direct Coupling with Arylboronic Acids.J. Am. Chem. Soc. 2013, 135, 86–89. (Highlighted by Synfacts 2013, 9, 0417.; Highlighted by Chin. J. Org. Chem. 2013, 33, 654.) (6) Nimmagadda, S. K.; Liu, M.; Karunananda, M. K.; Gao, D.-W.; Apolinar, O.; Chen, J. S.; Liu, P.; Engle, K. M. Catalytic, Enantioselective α-Alkylation of Azlactones with Non-Conjugated Alkenes via Directed Nucleopalladation. Angew. Chem. Int. Ed. 2019, 58, 3923–3927. (5) O’Duill, M. L.; Matsuura, R.; Wang, Y.; Turnbull, J. L.; Gurak Jr, J. A.; Gao, D.-W.; Lu, G.; Liu, P.; Engle, K. M. Tridentate Directing Groups Stabilize 6-Membered Palladacycles in Catalytic Alkene Hydrofunctionalization. J. Am. Chem. Soc. 2017, 139, 15576–15579. (4) Wang, J.; Gao, D.-W.; Huang, J.; Tang, S.; Xiong, Z.; Hu, H.; You, S.-L.; Zhu, Q. Palladium-Catalyzed Enantioselective C(sp2)–H Imidoylation by Desymmetrization. ACS Catal. 2017, 7, 3832–3836. (3) Yin, Q.; Wang, S.-G.; Liang, X.-W.; Gao, D.-W.; Zheng, J.; You, S.-L. Asymmetric Dearomative Chlorination of β-Naphthols. Chem. Sci. 2015, 6, 4179–4183. (2) Shi, Y.-C.; Yang, R.-F.; Gao, D.-W.; You, S.-L. Enantioselective Synthesis of Planar Chiral Ferrocenes via Palladium-Catalyzed Annulations with Diarylethynes. Beilstein J. Org. Chem.2013, 9, 1891–1896. (1) Peng, J.; Zong, C.; Ye, M.; Chen, T.; Gao, D.-W.; Wang, Y.; Chen, C. Direct Transition-Metal-Free Intramolecular C–O Bond Formation: Synthesis of Benzoxazole Derivatives. Org. Biomol. Chem. 2011, 9, 1225–1230. BOOK CHAPTER (1) Gao, D.-W.; Zheng, J.; Ye. K.-Y.; Zheng. C.; You, S.-L. Asymmetric Functionalization of C–H Bonds via a Transient Carbon-Metal (C–M) Species. in Asymmetric Functionalization of C–H Bonds, You, S.-L., Ed., Royal Society of Chemistry 2015, pp.141-213. PATENTS (1) You, S.-L.; Gao, D.-W.; Gu, Q. A Kind of Planar Chiral Ferrocene Compounds, Synthetic Methods and Their Applications. The Number of Patent (ZL201510757648.8). (2) You, S.-L.; Gao, D.-W.; Yin, Q.; Gu, Q. The Preparation of Planar Chiral Ferrocene Derivatives and Their Applications. The Number of Patent (ZL201410040306.X). (3) You, S.-L.; Gu, Q.; Gao, D.-W.; Shi, Y.-C.; Zhao, Z.-L. The Synthesis of New Kind of Planar Chiral Ferrocene Compounds and Their Applications. The Number of Patent (ZL201210489246.0). |
Team |